Natural materials are some of the most impressive structures on Earth. Take the humble tendon, a material found in every human, holding together our muscles. This material is stronger than steel on a strength-to-weight basis, and yet it is woven from minuscule individual fibers.
There’s a lot to learn from nature’s designs. It has produced some of the most performant materials known to humankind. For this reason, the chemical tapestries composing these materials are of great interest to modern chemists and scientists.
Chemists have found, to their surprise, a great degree of similarity among materials found in the natural world — from plant fibers to wool, silks, starches, leather, protein, and even DNA. All of the above were produced with long chains of carbon-based compounds called polymers.
The wondrous properties of natural polymers enticed chemists to look for methods of producing synthetic polymers. Chemists wanted to mimic the properties of these naturally occurring materials and produce them at scale. In the 20th century, a cousin of organic polymers was born — a family of synthetic polymers that slowly began taking over the world. In layman’s terms today, we call these synthetic polymers plastics.
The Ubiquity of Plastics
Over the past couple of decades, plastics have gotten a bad reputation. Nearly all plastics are currently produced from petroleum and thus are closely intertwined with the fossil fuels industry. Beyond that, estimates suggest that nearly half of all plastics produced each year are single-use, bound eventually for the landfill. In recent years, the sheer scale of non-biodegradable waste produced by plastic products has caused notable harm to the environment. Not only is there a continent of plastic garbage floating around in the middle of the Pacific, but plastic bags and wrappers have been found in the deepest recesses of the oceans. If that’s not enough, microplastics now seem to be invading everything from the air we breathe to our water supply.
As a result, the very term ‘plastic’ now carries a pejorative, wasteful, and even ugly connotation. However, the reality is that plastics, or synthetic polymers, as they are more diplomatically called, make the world go round.
Synthetic polymers are ubiquitous and useful. You put synthetic polymers in your hair and on your face in the form of shampoos and lotions, you brush your teeth with synthetic polymer fibers in the form of toothbrushes, and you go to bed warm at night because of the synthetic polymers in your duvet and home insulation. Dialysis patients rely on synthetic polymer membranes that can filter their blood, and entire regions depend on semi-permeable polymer membranes to filter salt out of their water supplies in desalination plants. All this merely scrapes the surface of their many applications.
The Composition of Synthetic Polymers
Each of the materials alluded to above is produced by stringing together a handful of carbon-based molecules called monomers. Carbon is the key here — its atomic structure makes it easy to chain to other atoms. It therefore serves as a strong and extensible backbone for nearly all organic matter. The production of specific polymers involves controlling how units of carbon monomers are chained together.
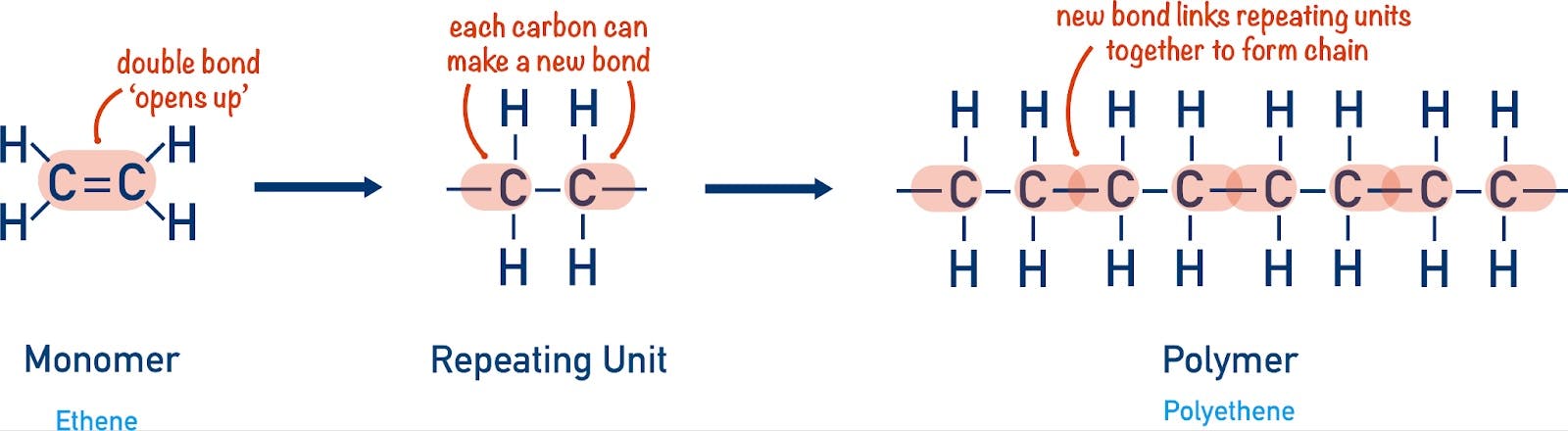
Source: Chemistry Student
At present, the most abundant source of carbon monomers is petroleum. This makes sense since petroleum is decayed organic matter that has been broken down over millions of years by natural forces. It’s essentially a soup of all the basic ingredients we could need to produce our own carbon macromolecules.
Each year, nearly 500 million tons of plastics are produced, making the polymer industry larger than the copper, steel, and aluminum industries combined. Outside of concrete, plastic is the most commonly used material in the world.
Though the United States might not be known as a prominent exporter of goods, when it comes to plastics, America is one of the world leaders. Plastics are produced at petrochemical plants. Typically, these are located very close to crude oil or natural gas extraction sites, which means that in the United States, one of the most geographically convenient locations for such plants is in Texas. Due to the the mature oil industry there, and the ease of transport provided by the Gulf of Mexico, the shores of Texas and Louisiana are home to the majority of petrochemical facilities in America.
The vast majority of petroleum produced is used as fuel. Less than 10% of all petroleum extracted goes towards producing petrochemicals. Of this fraction, half goes towards the creation of solvents like paints and adhesives, and the other half goes towards producing monomers.
Since petroleum carries long strands of carbon macromolecules, extracting the monomers requires a process called “cracking”. This is where liquid petroleum is heated to break the bonds of the larger macromolecules, leaving behind individual compounds like ethylene, propylene, and styrene as products.
Next, these monomer compounds go into a reactor, where the monomers react with each other one at a time to construct long polymer chains. This process happens in a large solution, and it’s worth noting that by the end of it, there are often still monomers left over. It’s why most plastic objects we use still likely have trace amounts of monomers on them. The imperfections of the polymerization process, in part, are what’s responsible for the proliferation of small microplastics in the environment.
A monomer like ethylene, for instance, is strung together into polyethylene, the most commonly used plastic in the world. Polyethylene is used in everything from plastic bags to milk containers to cable sheaths. Propylene becomes polypropylene, which is notable for its durability and heat resistance, and is found in everything from car parts to solar panels.

Source: Testbook
Of course, this is just scratching the surface of all possible synthetic polymer products. In reality, there are over 900 different petrochemical facilities in the US that are devoted to making products ranging from polystyrene (also known as styrofoam), to polyester, polyvinyl chloride (PVC), to polycarbonate (plexiglass), to polytetrafluoroethylene (teflon), to polyaramids (kevlar), polyamides (nylons), and more.

Source: EPA
The reason there are nearly a thousand plants dedicated to producing plastics of all kinds is that plastic is an incredibly versatile material. In the first place, polymer densities are very low, which means that relative to other materials, like metals, they are incredibly lightweight.
At the same time, however, plastics can be incredibly durable. Anyone who has ever battled a tangle in their hair knows why: when polymers get long enough, they exhibit a characteristic called ‘entanglement’ where they essentially mesh and form covalent bonds with other polymer strands around them. The entanglement of polymers is what gives polycarbonate, or plexiglas, its resilient quality, and what makes it the material of choice for things like bullet-proof windows.
Aside from this, polymers take very little energy to produce. Though there might be some objection to their origins as petroleum byproducts, the actual process of polymer synthesis required to make final plastics takes 26 times less energy than the production of aluminum, five times less energy than the production of steel, three times less energy than the production of glass, and 2.5 times less energy than even the production of paper.
Easy to make means these miracle materials are also incredibly easy to mold, extrude, and shape into a variety of one-, two-, or three-dimensional shapes. Furthermore, nearly everything about the chemical composition of polymer chains can be controlled, from the exact monomers that go into the chains to the order in which they are arranged. Even the ultimate shapes the polymers create can be designed. You can branch the polymer chains or graft new chains onto existing ones. You can make star-like structures or tree-like structures. You can make these materials transparent, translucent, or opaque.
Beyond this, polymers can be made to insulate electricity or conduct it. They can even be semiconducting or magnetic. They can change state upon being prompted too. Under the presence of an electrical field, or when exposed to light or temperature changes, certain polymers can change color or go from transparent to opaque. This is the secret behind the operation of liquid crystals in liquid crystal displays.
Due to the seemingly infinite variability in material qualities you can achieve with polymers, polymer science sits at the very center of material science itself, and there is much that remains to be discovered about polymers and their use. But before looking into that future, it’s important to understand the emergence of synthetic polymers as a crucial material in the industrial era.
The History of Polymers
The story of polymers is closely tied to the story of life itself, although polymers predate the emergence of life. In some primordial pool millions of years ago, the miraculous synthesis of the first lipid bilayers and amino acids created the conditions for the first living beings. It’s the mystery of polymers’ first appearance on Earth with which all origin-of-life theorists are concerned. Solving the riddle of how spontaneous polymerization can happen in nature may also be the clue to understanding how life itself emerged.
Ever since its origin, polymers have been the chemical backbone for all life on Earth. Organic polymers physically make up all living matter, from the proteins of animals to the starches of plants. For thousands of years, naturally-occurring polymers have not only been feeding us, but through the use of cotton, wool, silk, and leather have been clothing us too.
One way to look at industrial history, then, is the process of replacing the use of these naturally occurring materials with artificial ones — specifically, the replacement of organic polymers with synthetic ones. One of the first instances of this came with the modification of rubber.
Natural rubber is native to the Amazon rainforest and was first used by indigenous populations like the Aztecs. Christopher Columbus reported the existence of rubber after his travels to South America. However, upon first discovery, the curious soft material melted too easily when heated and became too brittle when cold and therefore found few useful applications.
Then, in 1770, a chemist named Joseph Priestly realized that the material was quite useful in removing graphite marks from paper and gave it a name that stuck: rubber. However, it would take until 1839 for rubber to find some other useful applications at last. That year, a self-taught American chemist named Charles Goodyear was experimenting with ways to improve the properties of rubber. He sought to keep its malleable properties while preventing it from melting too easily under heat and losing its shape.
By sheer accident, Goodyear managed to mix a sample of rubber and sulfur together under the presence of heat. It worked, and lo and behold, a stronger, more durable rubber was the result. He called this new creation ‘Vulcanite.’ This was the first successfully modified natural polymer, which in sixty years would be featured on the wheels of every car in the world. Though Goodyear himself didn’t live long enough to see this, the Goodyear tire company was named after him in the US when it began operations in 1898.

Source: Goodyear
The first truly synthetic polymer, however, was a product called bakelite. In 1907, Leo Baekeland, a successful chemist operating out of Yonkers, was experimenting with combinations of phenol and formaldehyde, both small carbon-based monomers, to see if he could produce a synthetic resin. At that time, shellac was one of the only substances capable of finishing and sealing wood.
In a world where everything was made of wood, shellac was a very valuable commodity. The only problem was shellac was produced by the secretions of a special bug found in India, so procuring it was tedious, to say the least. Though the phenol-formaldehyde mixture didn’t quite yield a new resin, it produced something even more valuable: the first-ever plastic.
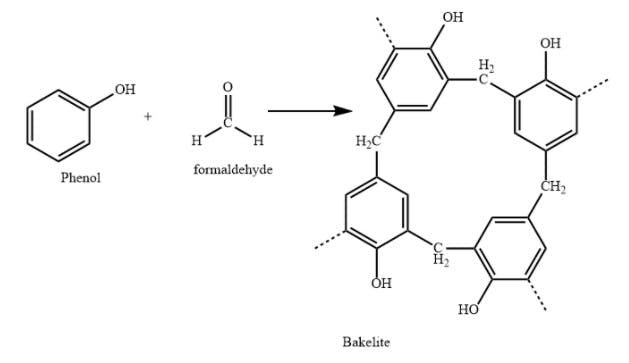
Source: Vedantu
Bakelite was quite a miraculous material for the early 20th century. It was unlike anything seen before at the time. It was produced by heating two simple ingredients, but once it set, it would not melt. It was entirely waterproof and didn’t corrode under the presence of any acids. Within a decade, bakelite was everywhere. It transformed American manufacturing and industrial design as bakelite was being used to make everything from radios and telephones to toothbrushes, combs, billiard balls, and dominoes.

Source: This Belongs in a Museum
Still, most chemistry in this era amounted to a very sophisticated regime of trial and error. At the time, there was little fundamental understanding of what was physically going on at the level of molecules in many of these polymer compounds, natural or otherwise. The best guess, which became the conventional view, was that polymers like starches or cellulose were big colloids, meaning they were essentially made of blobs of molecules suspended firmly in solution.
It was a chemist named Hermann Staudinger, however, who first proposed an opposing view to this theory. Staudinger wrote that polymers weren’t colloids, but actually long chains of repeating units. This theory became known as “chain theory”. It helped chemists deduce the structure of polyethylene and correctly hypothesize the way in which polymerization takes place. Though for many years Staudinger was ridiculed for these ideas, he was ultimately vindicated when chain theory was proven correct, eventually leading him to be awarded the Nobel Prize for his discovery in 1953.
In the decades that followed, the United States, soon followed by the rest of the world, underwent a synthetic fiber spring led primarily by the Du Pont corporation. Du Pont’s historical legacy was solidified primarily by one brilliant chemist it hired named Wallace Carothers. Carothers was fascinated by Staudinger’s ideas and figured that if they were true, macromolecules could be rationally synthesized by building longer chains from smaller units. Du Pont soon became Carothers’s laboratory. In 1935, Carothers discovered nylon. Nylon was a polyamide that quickly took over the fashion world by successfully mimicking silk. Another Carothers invention, which also took place at Du Pont, was neoprene, which was one of the first fully synthetic rubbers.

Source: Science History Institute
During the Second World War, the military was one of the plastic industry’s largest customers. Impervious materials like plexiglass proved useful replacements for brittle glass in military assets like jets. By the 1950s, all kinds of new plastic products were being released, from polystyrene to polyethylene to teflon.
However, after the end of the war, the plastics industry needed to replace its sudden fall in demand and it began thinking up new civilian applications. Nylon went from the material used to make parachutes to the material used to make women’s stockings. Women hosted Tupperware parties to sell the elusive and exciting new material for longer food storage. From the 1950s, the production of plastics doubled every decade, and by 1979, the world was producing more plastic than steel.
The Future of Synthetic Polymers
The kinship between plastics and organic matter is perhaps what is most fascinating about them. It’s also what makes synthetic polymers so versatile and powerful. It’s in studying the structure of naturally occurring matter and polymers that we developed the ambition to mimic natural materials and one day perhaps surpass them.
James Gordon, considered one of the founders of material science summed up his philosophy on materials and engineering nicely in the following quote from his book ‘Structures, or Why Things Don’t Fall Down’:
“The lilies of the field toil not, neither do they calculate, but they are probably excellent structures, and indeed Nature is generally a better engineer than man. For one thing, she has more patience and, for another, her way of going about the design process is quite different.”
Learning the structure of Nature’s designs, argued Gordon, is the best education an engineer could hope for. The structures of trees, muscles, and tendons are ingenious because they have had eons to develop. Of course, the ambition of all chemists is to push our ability to synthesize new compounds to the limit.
Outside of polymers already being used to help us study organic tissues like proteins — useful for everything from producing artificial meat to learning the keys to growing organs — researchers are now experimenting with ways they might be able to program the behavior of polymers. We already know that polymers can react to physical perturbation, light, and changes in temperature. Now, the question is whether these qualities can help synthesized polymers adopt pre-programmed behaviors.
Programmable polymers are already being explored in fields like organic electronics. However, other valuable applications can be imagined too. Picture a polymer treatment that when introduced to the body could target and destroy malignant cancer cells. Still others are exploring ways to produce self-healing polymers. Imagine what it might be like to have clothes that repair their own tears!
Other frontier research is exploring how polymers can be used to store information at the molecular level. We already know DNA does this, but if researchers succeed in producing an information storage function alongside programmability, it would allow us to produce a material capable of computing. This is, of course, a desired step along the road to the holy grail of material and life sciences: self-organizing matter.
However, before we get there, polymer science is sure to yield many more fascinating materials. Already, one contemporary descendant of work on synthetic polymers is graphene. Though not technically a polymer itself, graphene is one of the most impressive new carbon-based materials on the block. It’s a flat, or two-dimensional lattice made entirely of carbon atoms. Essentially, it’s a one-atom-thick sheet of diamond. The properties of graphene are stunning. It’s one of the lightest materials, and yet with a tensile strength ratio greater than steel and kevlar. Not to mention, it’s also one of the best conductors of electricity.
Despite all the wonders afforded by the production of polymers, these achievements have come at a high cost. Polyethylenes, styrofoams, and all other single-use plastics are not biodegradable. Throwing them out has produced an incredible swell of pollution throughout of natural environment, one which will not go away by itself for another thousand years. It’s not only wildlife that is being impacted, but humans everywhere are beginning to experience the negative effects that come with having trace amounts of monomers everywhere. The disruption that carbon-based compounds like BPA or bisphenol A, can cause to the human endocrine system is already a widespread cause of concern.
Whatever the future of polymer science, a great degree of its efforts will go toward discovering alternative plastic regimes — whether that means finding ways by we could accelerate the dissolution of plastics, like via plastic-eating bacteria, re-engineering plastics to make them more biodegradable, or finding non-fossil fuel based feedstocks to produce them.
In the realm of material science, polymer science stands at its core, driving much of the modern world's material innovation. These versatile, powerful materials are everywhere, molding the products we use daily. However, their widespread use has brought about significant downsides — waste and pollution are now pressing issues. While the highest ambitions for new polymer materials could completely transform what we thought materials were capable of, over the coming decades we will need to simultaneously grapple with new methods that can clean up the modern mess caused by the age of polymers.
Disclosure: Nothing presented within this article is intended to constitute legal, business, investment or tax advice, and under no circumstances should any information provided herein be used or considered as an offer to sell or a solicitation of an offer to buy an interest in any investment fund managed by Contrary LLC (“Contrary”) nor does such information constitute an offer to provide investment advisory services. Information provided reflects Contrary’s views as of a time, whereby such views are subject to change at any point and Contrary shall not be obligated to provide notice of any change. Companies mentioned in this article may be a representative sample of portfolio companies in which Contrary has invested in which the author believes such companies fit the objective criteria stated in commentary, which do not reflect all investments made by Contrary. No assumptions should be made that investments listed above were or will be profitable. Due to various risks and uncertainties, actual events, results or the actual experience may differ materially from those reflected or contemplated in these statements. Nothing contained in this article may be relied upon as a guarantee or assurance as to the future success of any particular company. Past performance is not indicative of future results. A list of investments made by funds managed by Contrary (excluding investments for which the issuer has not provided permission for Contrary to disclose publicly as well as unannounced investments in publicly traded digital assets) is available at www.contrary.com/investments.
Certain information contained in here has been obtained from third-party sources, including from portfolio companies of funds managed by Contrary. While taken from sources believed to be reliable, Contrary has not independently verified such information and makes no representations about the enduring accuracy of the information or its appropriateness for a given situation. Charts and graphs provided within are for informational purposes solely and should not be relied upon when making any investment decision. Please see www.contrary.com/legal for additional important information.



